How NATPARA Is Given
Patients and caregivers who will reconstitute and administer NATPARA should receive appropriate training and instruction prior to first use.
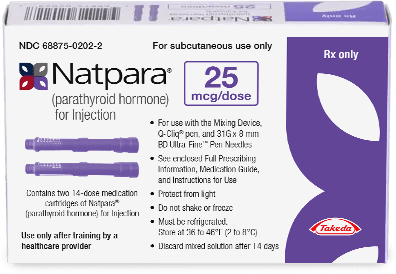
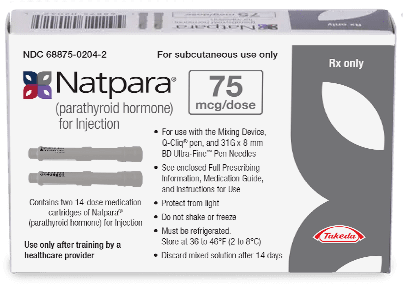
How NATPARA is supplied
NATPARA is supplied as a multiple dose, dual-chamber glass cartridge containing a sterile powder and diluent in 4 dosage strengths:


Reconstitution and Administration of NATPARA
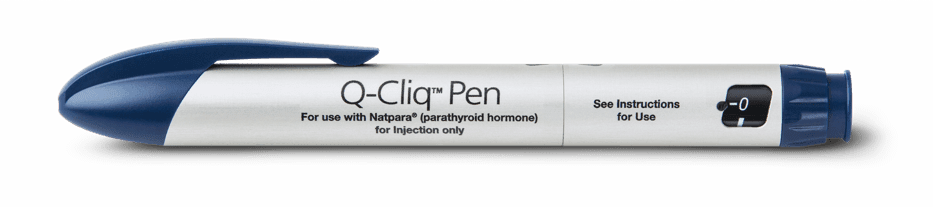
- NATPARA Medication Cartridges are reconstituted with the Mixing Device and administered with the Q-Cliq™ pen injector.
- Prior to administration, the NATPARA Medication Cartridge should be visually inspected for particulate matter and discoloration.
- Each NATPARA Medication Cartridge can be used for 14 subcutaneous injections. Patients should alternate between thighs each time they inject.
- Following each administration, the disposable needles should be discarded in a puncture-resistant container.
- NATPARA Medication Cartridges should be stored in the refrigerator (36°F to 46°F [2°C to 8°C]). Q-Cliq™ pens that still contain doses should be stored in a refrigerator. All reconstituted NATPARA Medication Cartridges older than 14 days must be discarded.
Severe hypercalcemia has been reported with NATPARA. The risk is highest when starting or increasing the dose of NATPARA.1
Reference: 1. NATPARA [package insert]. Shire-NPS Pharmaceuticals, Inc.

