Dosing Guidelines for NATPARA1
NATPARA is a self-administered, once-daily, subcutaneous injection into alternating thighs.1 The goal of NATPARA treatment is to achieve serum calcium within the lower half of the normal range.
Before initiating NATPARA and during treatment with NATPARA
- Confirm 25-hydroxyvitamin D stores are sufficient. If insufficient, replace to sufficient levels per standard of care
- Confirm serum calcium is above 7.5 mg/dL before starting NATPARA
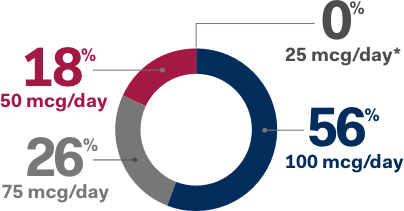
INITIATE NATPARA
ADJUST VITAMIN D & CALCIUM
TITRATE DOSAGE
MONITOR TREATMENT
Interruption or Discontinuation
Abrupt interruption or discontinuation of NATPARA can result in severe hypocalcemia. Resume treatment with, or increase the dose of, an active form of vitamin D and calcium supplements if indicated in patients interrupting or discontinuing NATPARA, and monitor for signs and symptoms of hypocalcemia and serum calcium levels. In the case of a missed dose, the next NATPARA dose should be administered as soon as reasonably feasible and additional exogenous calcium should be taken in the event of hypocalcemia. For full dosage and administration instructions, please see the full Prescribing Information section 2.
Reference: 1. NATPARA [package insert]. Shire Pharmaceuticals, Inc.