Understanding Hypoparathyroidism
Hypoparathyroidism interferes with calcium homeostasis2
Hypoparathyroidism is a complex endocrine disorder characterized by absent or inappropriately low levels of parathyroid hormone (PTH). Compromised endogenous PTH function can lead to hypocalcemia.2-4
Calcium levels can be maintained through interactions that involve endogenous PTH and the bones, kidneys, intestines, and the active form of vitamin D2
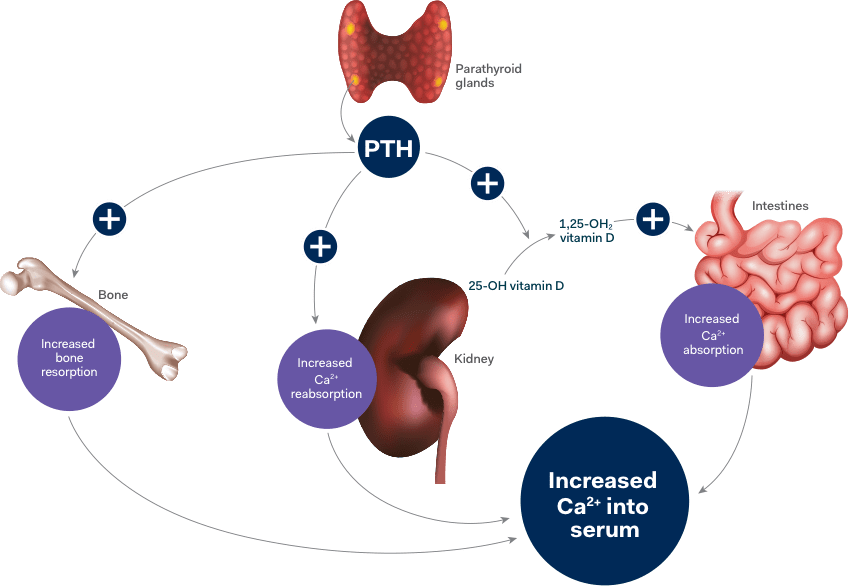
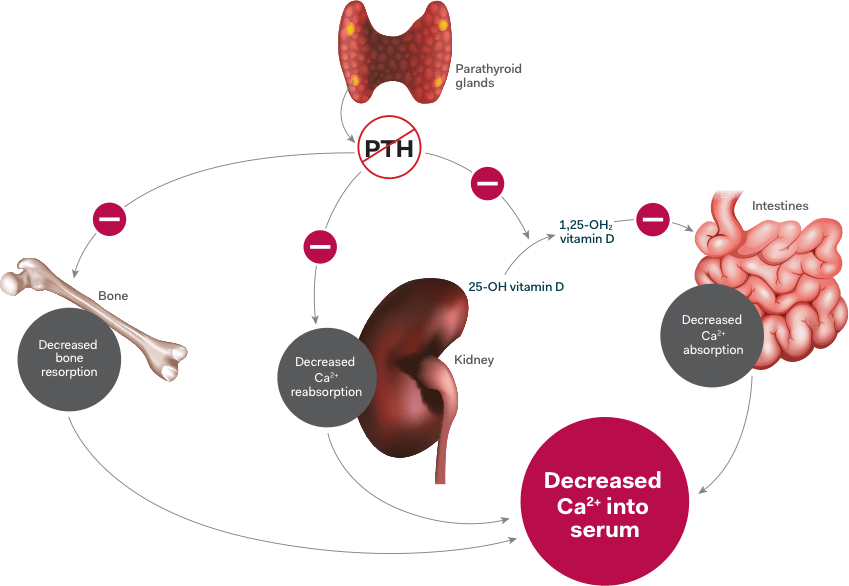
Insufficient endogenous PTH can interfere with certain processes of these organs which can lead to2,3:
- Decreased intestinal calcium absorption due to a decrease in the conversion of vitamin D to its active form
- Decreased renal reabsorption of calcium
- Decreased bone turnover
References: 1. NATPARA [package insert]. Shire Pharmaceuticals, Inc. 2. Shoback D. N Engl J Med. 2008;359(4):391-403. 3. Bilezikian JP, Khan A, Potts JT Jr, et al. J Bone Miner Res. 2011;26(10):2317-2337. 4. Fong J, Khan A. Can Fam Physician. 2012;58(2):158-162.
