Understanding NATPARA
NATPARA’s active ingredient is a recombinant human parathyroid hormone (rhPTH)1
It contains 84 amino acids and has a terminal half-life of approximately 3 hours1
Amino acid sequence of PTH1

NATPARA is administered once per day1
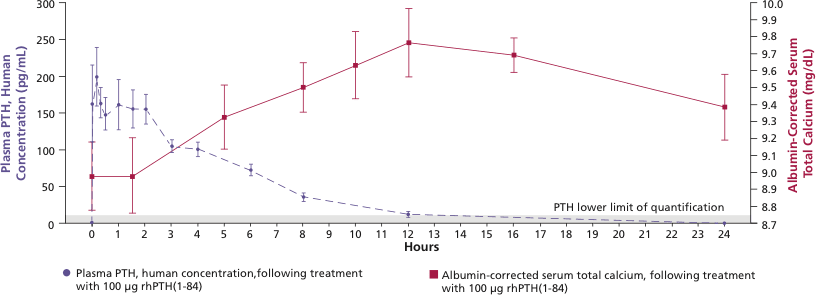
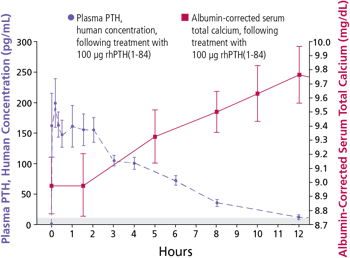
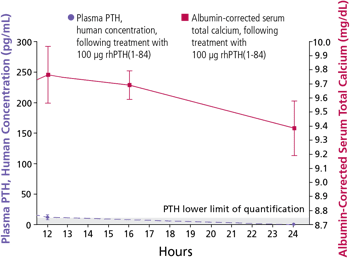
Mean (±SE) unadjusted PTH and albumin-corrected serum calcium concentration following 100 mcg SC administration in patients with hypoparathyroidism 1
Treatment with NATPARA increases serum calcium levels, which are sustained for more than 24 hours after administration.
Mean peak serum calcium levels are reached between 10 and 12 hours following a single subcutaneous injection, and the increase in serum calcium above baseline is sustained for more than 24 hours after administration.
Peak plasma concentrations of NATPARA occur within 5 to 30 minutes with a second, usually smaller, peak at 1 to 2 hours.
Osteosarcoma Boxed Warning
During animal drug testing, NATPARA caused some rats to develop osteosarcoma. The occurrence of osteosarcoma was dependent on parathyroid hormone dose and treatment duration. The rats received 3 different doses of NATPARA, producing drug levels in the body about 3 to 71 times the levels measured in humans who received the maximum approved dose. These data could not exclude a risk to humans. Because of a potential risk of osteosarcoma, use NATPARA only in patients who cannot be well-controlled on calcium and active forms of vitamin D alone and for whom the potential benefits are considered to outweigh this potential risk.
Instruct patients to promptly report signs and symptoms of possible osteosarcoma such as persistent localized pain or occurrence of a new soft tissue mass that is tender to touch.
Reference: 1. NATPARA [package insert]. Shire Pharmaceuticals, Inc.