Managing Hypoparathyroidism
Managing hypoparathyroidism requires careful monitoring2
In stable patients:
- Serum calcium, phosphorous/phosphate, magnesium, BUN/creatinine and eGFR at least yearly
- 24-hour urine collection for calcium and creatinine yearly
During dose adjustments:
- More frequent monitoring, often several times per week, until a stable serum calcium concentration is achieved
For dosing and monitoring specific to NATPARA, click here.
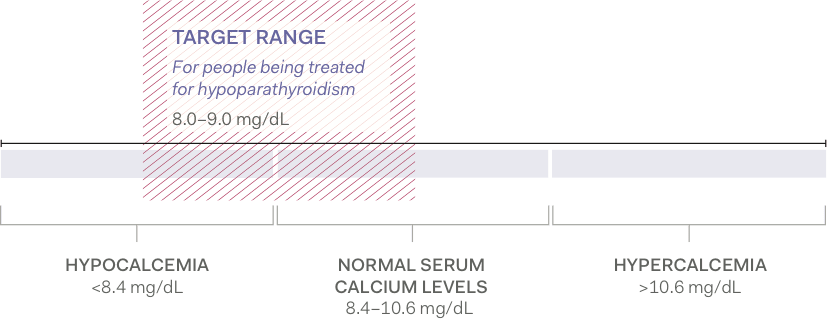
Aim for low-normal, albumin-corrected total serum calcium levels2,3
For patients being treated for hypoparathyroidism, serum calcium levels are typically maintained at the lower end of the normal range (approximately 8.0 to 9.0 mg/dL).2
Albumin-corrected total serum calcium levels1-4
References: 1. NATPARA [package insert]. Shire Pharmaceuticals, Inc. 2. Brandi ML, Bilezikian JP, Shock D, et al. J Clin Endocrinol Metab. 2016;101(6):2273-2283. doi: 10.1210/jc.2015-3907. 3. Shoback D. N Engl J Med. 2008;359(4):391-403. 4. Fong J, Khan A. Can Fam Physician. 2012;58(2):158-162.
